Healthcare Material Solutions
Arkema offers a wide range of medical grade resins for various healthcare applications.
Watch this video for a short introduction and overview of our medical product portfolio. Mohana, one of our Healthcare team engineers, will walk you through our medical product solutions and main end applications.
Download the Healthcare Solutions Presentation here.
Mohana, one of our Healthcare team engineers, will walk you through our medical product solutions and main end applications.
For more information on a specific medical product range, click on the brand names below.
Arkema's Healthcare High Performance Polymers Portfolio
All products have been tested for ISO 10993-4 and -5 and USP Class VI
(compliance letters available upon request)
Click here to download our medical products brochure
Click here to view Arkema's medical device policy
|
|
|
|
|
|
|
Take a deep dive into the Pebax® MED range of products and technical properties with Mohana, Business Development Engineer for Healthcare.
Download the Pebax® MED range presentation
Premier Material For Longer Length Catheter Shafts
Recognized for superior torque transference, minimal modulus change in the body, broad range of durometers, lowest hysteresis among TPEs, excellent kink resistance, chemical resistance and biocompatibility, Pebax® MED elastomers are the reference materials for PTCA (Percutaneous Transluminal Coronary Angioplasty) catheters, and are commonly used in other complex cardiovascular catheters, medical tubing and medical film applications.
The Pebax® MED grades range from soft, flexible and elastomeric properties to semi-rigid properties, similar to polyamides.
The remarkable processability of medical grade Pebax® elastomers make them an excellent choice for extrusion of medical grade tubing or film applications. They are easily compounded with medical additives to provide enhanced lubricity or radiopaque properties, among others..
Pebax® MV1074 SA 01 MED resin as an Additive for Permanent Antistatic Properties
Pebax® MV1074 SA01 MED resin can be used as a permanent antistatic additive (typically between 15% to 20% loading) in different matrices (ABS, COC, PMMA, HDPE, PP, etc.), to form a 3D antistatic network within the host matrix. This grade does not migrate to surface but rather produces an immediate and permanent antistatic effect at high and low humidities.
These properties are important for applications such as dry powder inhalers in which consistency of drug dosage delivery is critical.
Click here to learn more about medical antistatic applications.
Pebax® MV1074 SA01 MED for Breathable Films
The Pebax® MV1074 SA01 MED elastomer allows for the robust manufacture of breathable monolithic films compared to perforated or micro-porous film structures.
Pebax® MV1074 SA01 MED monolithic films offer adjustable MVTR, do not incur loss of performance over time, benefit from good tear strength, have excellent barrier properties, and high water-entry pressure.
This material is well suited for demanding medical gowns, innovative medical devices and pharmaceutical packaging, among other applications.
Pebax® MED grades TDS
Download the technical data sheets for Pebax® MED grades.
Discover brochures and presentations for Pebax® MED grades.
All literature for Pebax® MED grades
Take a deep dive into the Rilsan® MED & Rilsamid® MED product range and technical properties with Mohana, Business Development Engineer for Healthcare
Polyamides Performance Pyramid
Rilsan® MED Polyamide 11 grades are used in applications that require the strength and performance characteristics of a true thermoplastic, while also requiring sufficient flexibility and elongation. Rilsan® MED polymers are easy to process by most traditional methods, including extrusion, extrusion blow molding, injection molding, and rotomolding.
Rilsan® Polyamide 11 is the most advanced polyamide, offering improved elastic return properties and flexibility compared to other polyamides, biocompatibility, great chemical resistance, excellent resistance to sterilization techniques (steam, gamma, ETO), lightweight, and superior dimensional stability.
Rilsan® MED products are 100% bio-based advanced materials, derived from the castor plant. Polyamide 11 was developed over 70 years ago and Arkema remains the only producer in the world to master this product. Arkema is strongly committed to the continued development of Polyamide 11 as this material is the backbone of its High Performance Polymers strategy.
Arkema is a founding member of the Pragati initiative, which encourages and educates farmers in India to sustainably harvest castor and increase their standard of living.
For more information about Pragati, click here.
Interested in learning why Rilsan® MED is a true advanced sustainable material and how it can fit your needs (or replace some of your non-sustainable advanced polymers?)
Submit a request through the contact form below to schedule a call with one of our Bio Ambassadors. Arkema’s Bio Ambassadors will provide you with a robust overview of the entire supply chain from castor farming to recyclability.
List of Rilsan® MED grades
|
Injection - Rilsan® BMNO MED – TDS |
Extrusion - Rilsan® BESNO MED – TDS |
|
Grade with negligible amount of oligomers – Rilsan® BESVO A MED – TDS |
Translucent copolyamide – Rilsan® 8020 – TDS |
DOWNLOAD OUR MEDICAL PORTFOLIO BROCHURE (FEATURING ALL GRADES AND TECHNICAL DATA SHEETS)
All literature for Rilsan® MED grades
Take a deep dive into the Rilsan® Clear MED product range and technical properties with Mohana, Business Development Engineer for Healthcare
Rilsan® Clear MED grades offer an outstanding combination of transparency, lightweight, chemical resistance, and flexibility used in applications such as BPA-free and BPS-free respiratory masks, medical tubing, and storage containers.
Transparent
- Light transmittance of 91% - exceeding that of glass and polycarbonate
Lightweight
- With density of 1.01 for Rilsan® Clear G850 Rnew® MED and 1.05 for Rilsan® Clear G170 MED, these transparent polyamides are way lighter than traditional transparent medical plastics such as acrylics, polycarbonates or PSU.
Flexible
- Flexural modulus of 1,980 MPa for Rilsan® Clear MED G170 polyamide and 1,600 MPa for Rilsan® Clear G850 Rnew® MED polyamide representing a strong improvement in flexibility compared to polycarbonate.
Durable
- High temperature resistance, good surface and wear resistance, good chemical resistance, BPA and plasticizer free and sterilizable
Bio-based option
- Rilsan® Clear G850 Rnew® MED is 45% bio-based (derived from the castor plant)
Take a deep dive into the Rilsan® MED & Rilsamid® MED product range and technical properties with Mohana, Business Development Engineer for Healthcare
Rilsamid® MED polyamide 12 grades are used in medical applications that require the strength and performance characteristics of a true thermoplastic, while also requiring sufficient flexibility and elongation. Rilsamid® MED polymers are easy to process by most traditional methods, including extrusion, extrusion blow molding, injection molding and rotomolding.
Rilsamid® Polyamide 12 is an advanced polyamide, offering biocompatibility, great chemical resistance, excellent resistance to sterilization techniques (steam, gamma, ETO), lightweight and dimensional stability.
List of Rilsamid® MED grades
| Injection - Rilsamid® AMNO MED – TDS | Extrusion - Rilsamid® AESNO MED – TDS |
Download the full MED range presentation
All literature for Rilsamid® MED grades
Take a deep dive into the Kynar® MED product range and technical properties with Mohana, Business Development Engineer for Healthcare
Kynar® MED PVDF is the newest addition to Arkema’s High Performance Polymers medical portfolio.
Kynar® MED PVDF can easily be processed on conventional equipment. A large processing window allows Kynar® MED PVDF to be safely injection molded, extruded or injection blow molded. Kynar® MED PVDF exhibits excellent chemical resistance, high barrier properties, excellent abrasion resistance, high tensile strength and can be sterilized using Gamma, ETO and steam.
Comparison of the key mechanical properties of Kynar® MED PVDF to other fluoropolymers
| PROPERTIES | Kynar® 720 MED | PTFE | PFA | FEP | ETFE | ECTFE |
| Melting Point (°C) | 168 | 325 | 305 | 260 | 270 | 245 |
| Specific Gravity | 1.78 | 2.18 | 2.15 | 2.15 | 1.7 | 1.69 |
| Tensile Strength at Yield (psi) | 7,250 | 1,700 | 2,200 | 2,500 | 5,000 | 5,000 |
| Elongation at Break (%) | 100 | 300 | 300 | 325 | 250 | 200 |
| Hardness (Shore D) | 78 | 50 | 60 | 55 | 75 | 75 |
| Flexural Modulus (x10-3 psi) | 268 | 30 | 100 | 90 | 200 | 240 |
| Tabor Abrasion (mg/1000 cycles) | 5 | 60 | 25 | 75 | 60 | 7 |
| COF | 0.2 - 0.35 | 0.1 | 0.2 | 0.2 | 0.4 | 0.3 |
| Heat Deflection Temperature (°C) 66 psi |
133 |
120 |
75 |
70 |
105 |
125 |
| 264 psi | 110 | 55 | 50 | 50 | 75 | 75 |
The combination of low permeation and excellent chemical resistance lends this product line to be suitable for medical and pharmaceutical packaging applications
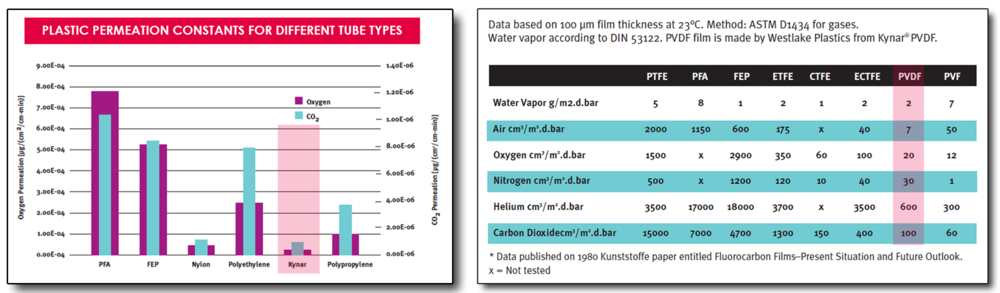
List of Kynar® MED grades
Homopolymer - Kynar® 720 MED – TDS






